When There’s a Will – There’s a Way
Our team is committed to improving the lives of cancer patients.
Introduction
Because GLIOBLASTOMA BRAIN cancer shouldn’t be a death sentence
Global Cancer Technology is a non-revenue biopharma company developing a novel treatment for cancers. We are developing a small molecule PI3K inhibitor that can cross the blood-brain barrier and has shown great promise in preclinical cell and animal studies as a potential glioblastoma therapy.
Glioblastoma Multiforme (GBM) is the most common form of brain cancer with an incidence rate of 3.21 per 100,000 population.
It is also the most malignant and aggressive form of brain tumor.
Most GBM’s have strong drug resistance against the PI3K/AKT/mTOR inhibitors due to the cytoprotective effect and the adaptive response of autophagy during the treatment.
Standard GBM treatments are toxic and associated with poor prognosis.
- Radiation can affect surrounding normal brain tissue
- Chemo has systemic adverse effects

Dr. Santosh Kesari
World-renowned Neuro-Oncologist-John Wayne Cancer Insitute
New Data! Glioblastoma Tumor Growth is Arrested
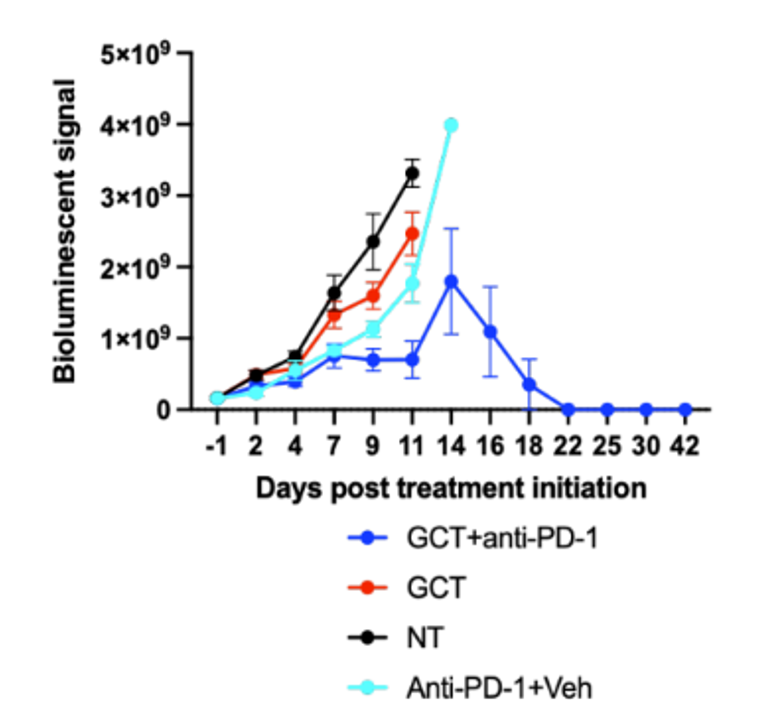
Our first anti-cancer inhibitor (patent pending) is currently in pre-clinical trials. Tumor growth in mice is arrested and the tumor appears to degrade based on the latest data.

- 15/052/526-Scintillating Nano-Crystals
- SD2013-199-, SD2016-342 "Wide field low dose irradiation to Activate an Ant-Tumor Pro-Drug carried by a Nano-vehicle", and "modification for improved PK"
The Problem
GBM's Unique Treatment Challenges
PI3K-AKT pathway is upregulated causing
Resistance to
- - Chemotherapy
- - Radiation
- - Immunotherapy
1
Suppression of
- - Immune Molecules
- - Lysosome Biogenesis
2
Activation of Microglia with the production of
- - Cytokines
- - Toxic Reactive Oxygen Species
3
Solution
OUR Solution: GCT-007
A unique, mechanistically distinct dual inhibitor targeting both the PI3K and mTOR pathways.
- Targets a novel PI3K pathway – autophagy
- Eliminates the problem of signaling blockade with conventional PI3K inhibitors by targeting more than one signaling protein
- Modulates immune cell function and inflammatory responses
- Crosses the Blood-Brain Barrier (BBB)
GCT-007 has thus far proven to:
- Induce Growth Arrest
- Increase Immune Recognition
- Increase Sensitivity to Radiation
Timeline
Research takes time and money. Bringing a drug to market now costs an average of $ 2.7 Billion and can take up to a decade in time. It is extremely challenging and with less funding, it can take even longer. Yet the potential to change lives makes it an attractive pursuit despite the complexity and risks.
GCT goal is to have a Phase 1 Breast Cancer + a Phase 2 Glioblastoma drug candidate by the end of 2027 with a targeted company valuation of $200 million.
*NOTE* that this is the goal – invest at your own risk.
GCT 007 Development Plan

Patent/Licenses/IP
GCT has acquired the right/license to the following the patents:
GCT-007: International Publication Number WO 2019/199864 A1 >TRI-SUBSTITUTED ARYL AND HETEROARYL DERIVATIVES AS MODULATORS OF PI3-KINASE AND AUTOPHAGY PATHWAYS > Issued in the US, Japan & China .. Other countries pending. Patent prosecuted by Morrison Forester LLP.
GCT-008: International Publication Number WO 2019/199874 A1 > MORPHOLINE DERIVATES AS INHIBITORS OF VPS34 > Patent Pending. Patent prosecuted by Morrison Forester LLP.
GCT has filed its patent for using GCT-007 with other checkpoint inhibitors > International application No.PCT/US2023/065433 > filed April 2023. Patent prosecuted by Wolf Greenfield LLP.
Patent for combining GCT-007 with GCT-008 filed March 2025. Patent prosecuted by Wolf Greenfield LLP.
